 |
 |
- Search
| J Environ Anal Health Toxicol > Volume 26(4); 2023 > Article |
|
ABSTRACT
A novel high performance chromatographic method was devised to simultaneously quantify chlorine dioxide and hypochlorous acid/hypochlorite, leveraging their similar chemical properties but distinct toxicities. In this study, it consists of a two-step derivatization reaction: The first step is the reaction of hypochlorous acid/hypochlorite with 2,6-dimethylphenol in a water matrix to produce 4-chloro-2,6-dimethylphenol; the second step is a reaction in which iodine ions added after the first step react with chlorine dioxide to produce iodine and then react with 2,6-dimethylphenol to produce 4-iodo-2,6-dimethylphenol. It was confirmed that the concentration of 4-chloro-2,6-dimethylphenol produced was proportional to the total concentration of hypochlorous acid/hypochlorite, and the concentration of 4-iodo-2,6-dimethylphenol was proportional to the concentration of chlorine dioxide. With a detection limit of 0.011 mg/L for chlorine dioxide and 0.009 mg/L for hypochlorous acid/hypochlorite, the calibration curve showed excellent linearity (r2=0.999) in disinfectant solution. This sensitive and selective method is well-suited for the routine analysis of chlorine dioxide and hypochlorous acid/hypochlorite in water and disinfection byproducts.
Chlorine dioxide was discovered in 1811 by Sir Humphery Davy, who named it greenish-yellow gaseous chlorine [1]. Chlorine dioxide is readily soluble in water to form a disinfectant solution against a wide range of microorganisms and is used as a disinfectant and oxidizer in drinking water, as well as to significantly reduce odor and color [1,2]. To date, its scope of application has been expanded to food processing, building and vehicle disinfection, mold removal, air disinfection and odor control, swimming pool treatment, dental applications, and wound cleaning [1-3].
Sodium hypochlorite (NaClO) is probably the world's most commonly used disinfectant. The WHO has recommended the use of 70% ethanol or 1000 mg/L sodium hypochlorite for disinfection of SARS-CoV-2 transmitted by aerosols and droplets [4]. Several studies have found that the concentration of chlorine dioxide is 10 times lower than the concentration of sodium hypochlorite required to inactivate the same amount of influenza A virus under the same conditions [5-7]. Studies have shown that chlorine dioxide is a more effective disinfectant against SARS-CoV-2 than sodium hypochlorite, even in the presence of organic matter, and chlorine dioxide is a strong disinfectant against SARS-CoV-2 and may be useful in reducing SARS-CoV-2 infection [8].
Meanwhile, the antiviral effect of chlorine dioxide against novel Coronavirus disease 2019 (COVID-19) has been overestimated and misused by suppliers. An Internet company sold Miracle Mineral Solution (MMS), which is sodium chlorite dissolved in distilled water. This product instructs people to mix the solution with citric acid, lemon juice or lime juice before drinking [9]. The USFDA has warned sellers of the risks of selling MMS for the prevention and treatment of COVID-19 consumers not to buy or drink chlorine dioxide products marketed as pharmaceuticals online [10]. Sodium chlorite reacts with the specified acid to form chlorine dioxide. It is not known how much chlorine dioxide and hypochlorite are produced when this product is mixed with citric acid, lemon juice, or lime juice.
Chlorine dioxide and hypochlorous acid hypochlorite are produced together during the production of chlorine dioxide. Due to the similar chemical properties of the two disinfectants, simultaneous and separate analysis of them is very difficult. Despite the difficulty of analysis, the two disinfectants have different toxicities, so the content of each disinfectant must be separately stated.
Several analytical methods based on different instruments have been published for the determination of chlorine dioxide. Spectrophotometry has been used to determine chlorine dioxide in water using dyes [11-16]. Ion chromatography (IC) method [17]. and high-performance liquid chromatography (HPLC) method [18] have also been reported for chlorine dioxide measurements. Additionally, a gas chromatography-mass spectrometry (GC-MS) method based on derivative reactions has been developed [19].
A post-column derivatization method using 4-aminoantipyrine and phenol after separation on an anion exchange column has been published for the simultaneous analysis of chlorine dioxide and hypochlorite. This method has high detection limits of 0.2 and 10 mg/L for chlorine dioxide and hypochlorite, respecti-vely [18]. Considering that the US EPA has set a maxi-mum of 0.8 mg/L for chlorine dioxide and 4.0 mg/L for hypochlorous acid [20,21], this method is not sensitive enough to simultaneously measure both disinfectants in drinking water.
The purpose of this study is to develop an analysis method that can simultaneously measure chlorine dioxide and hypochlorous acid/hypochlorite in water, and to apply the developed method to analyze the contents of chlorine dioxide and hypochlorite in chlorine dioxide disinfection products such as MMS.
Potassium iodide, sodium hypochlorite, sodium chlorite, 2,6-dimethylphenol (2,6-DMP), 4-chloro-2,6-dimethylphenol (4-chloro-2,6-DMP), 4-iodo-2,6-dimethylphenol (4-iodo-2,6-DMP), 2,4,6-trichlorophenol (2,4,6-TCP), ascorbic acid, hydrochloric acid and citric acid were obtained from Sigma/Aldrich (St. LOUIS, MD, USA). Sodium sulfate was purchased from Junsei (Tokyo, JPN). Ethyl acetate, methanol and acetonitrile (HPLC grade) were obtained from Honeywell (Charlotte, NC, USA). Pure water was purified with a Milli-Q Reagent-Grade water system (ZD20) and its resistivity was greater than 17 MΩ. Lemon and lime juice were obtained from a market in Kongju, Republic of Korea.
To derivatize hypochlorous acid/hypochlorite, 50 μL of a 5% 2,6-DMP solution (in MeOH) was added to a 5 mL sample or fortified sample and the solution was mechanically shaken for 5 min at room temperature. For the chlorine dioxide derivatization reaction, 20 μL of pure 5% potassium iodide was added to the mixed solution and mechanically shaken at room temperature for 20 min. Then, 100 μL of internal standard (10 mg/L 2,4,6-TCP in acetonitrile) was added to the sample solution. If the concentration of the analyte is in mg/L or if the analyte is only required to the mg/L level, the mixture is measured directly using HPLC-UV. On the other hand, if measurement in μg/L is required, the extraction and concentration is required as follows. The sample mixture was adjusted to pH 5 by adding 0.5 mL of acetate buffer and 2 mL of ethyl acetate as extraction solvent and 300 mg of sodium sulfate for salting out were added in the sample, followed by mechanical shaking for 10 min. After the solution was centrifuged at 150 rpm for 5 min, the organic layer was transferred to a 15 mL test tube. To prevent loss of derivatives during the concentration process, 50 μL of pure water was added to the organic extract and concentrated with a nitrogen stream to remove the organic solvent until only the water layer remained. The residue was mixed with 50 μL of acetonitrile and the extract was transferred to a V-shaped vial for measurement using HPLC-UV.
Calibration curve was established by adding the concentration range in Table 1 of 4-chloro-2,6-DMP, 4-iodo-2,6-DMP and 100 μL of 10 mg/L 2,4,6-TCP (in acetonitrile) in a 5.0 mL water. The ratio of the peak area of standard to that of internal standard was used in the quantitation of the compounds. The standard calibration curve was made by calculating the regression line of the peak area ratios of 4-chloro-2,6-DMP and 4-iodo-2,6-DMP to the internal standard against the analyte concentration.
Fortified samples were prepared at two concentrations for 4-chloro-2,6-DMP and 4-iodo-2,6-DMP, and recovery was calculated as the percentage of derivative recovered. The limit of detection (LOD) and limit of quanti-fication (LOQ) were calculated from the values measured in 7 fortified samples added to 5.0 mL pure water at concentrations that were expected to be the detection limit, as Student's t-value of 3.143 (99% confidence level for seven replicates) and 10 times the standard deviation of 7 measurements. Precision and accuracy was calculated with using the analytes recovered. Intra-day precision and accuracy were evaluated using five independent determinations at two concentrations, and inter-day precision and accuracy were evaluated using the samples on three different days.
To prepare chlorine dioxide products such as MMS, sodium chlorite was added to 0.1 M citric acid, 0.05 M hydrochloric acid, lemon juice and lime juice at concentrations of 30 mg/L and 100 mg/L. After mixing, the solution was shaken mechanically at room tem-perature for 5, 10, 30 and 60 min, and at each of the afore mentioned time periods, a 5 mL aliquot of the solution was transferred to a 15 mL test tube and proceeded through the derivatization and extraction procedure.
All HPLC chromatograms were obtained with an Agilent 1200 series (Agilent Technologies, CA, USA) using UV detector (220 nm). The analytical column was an Eclipse Plus C18 (4.6 mm ID, 150 mm length, 3.5 μm particle size). Solution A of the mobile phase was the mixture solution of water and acetonitrile (40:60), and solution B was acetonitrile. In this case, LC elution was carried out by linear gradient from 10% B to 50% B in 4 min, to 70% B in 10 min, to 100% B in 11 min to 50% B in 13 min, and to 10% B in 15 min. The flow rate of the mobile phase was 1.0 mL/min at 30oC and injection volume was 5 μL.
Chlorine dioxide and hypochlorous acid/hypochlorite are unstable, reactive and oxidizing agents. Because these two substances have similar chemical properties, it is difficult to differentiate between the two substances themselves. Fortunately, chlorine in chlorine dioxide and hypochlorous acid/hypochlorite exhibit clear differences in the substitution reactivity of chlorine in the two compounds, and the differences can be used to analyze the two disinfectants on a simultaneous chromatogram via derivatization reactions.
The first strategy for the simultaneous analysis of chlorine dioxide and hypochlorous acid/hypochlorite is to use the direct substitution reactivity of hypochlorous acid/hypochlorite to 2,6-DMP. Chlorine in hypochlorous acid/hypochlorite undergoes a rapid SN2 sub-stitution reaction with hydrogen at the para position of 2,6-DMP to 4-chloro-2,6-DMP as shown in Scheme I, but chlorine in chlorine dioxide does not undergo a substitution reaction with hydrogen.
The second strategy is to use chlorine dioxide oxidation reactivity of iodide to iodine followed by substitution reaction with 2,6-DMP. After the hypochlorous acid/hypochlorite substitution reaction is completed, iodide ions should be added to the reaction solution. The residual chlorine dioxide can rapidly oxidize iodide to iodine, and then form I3-(Scheme II). Triiodide reacts to a rapid SN2 substitution reaction with hydrogen at the para position of 2,6-DMP to 4-iodo-2,6-DMP as shown in Scheme III.
The two products, 4-chloro-2,6-DMP and 4-iodo-2,6-DMP, can be easily analyzed by HPLC-UV. If trace amounts of chlorine dioxide and hypochlorite remain after the derivatization reaction, the concentrations of 4-chloro-2,6-DMP and 4-iodo-2,6-DMP may change, so it is recommended to use ascorbic acid as a quenching agent.
Using the above basic reaction process, optimal conditions for simultaneous analysis of chlorine dioxide and hypochlorite were established.
The reaction rate of hypochlorous acid/hypochlorite with 2,6-DMP was studied. The reaction rate was determined by the detection of the substituted product, 4-chloro-2,6-DMP, after the quenching of residual chlorine with ascorbic acid at reaction times of 1.0, 2.0, 3.0, 5.0, and 10 min. As a result, the 2,6-DMP showed a very rapid reaction with hypochlorous acid/hypochlorite. If enough 2,6-DMP is present in the reaction mixture, the reaction is completed in about 1 min at room temperature. After this period, the reaction yield remained constant.
The reaction rate of chlorine dioxide with iodide and 2,6-DMP was studied. This sample was analyzed at reaction times of 0, 1.0, 2.0, 5.0, 10, 20 and 30 min. In the presence of a sufficiently high concentration of 2,6-DMP, the reaction is completed in about 10 min at room temperature without significant change in reaction yield.
Therefore, the simultaneous analysis of chlorine dioxide and hypochlorous acid/hypochlorite consisted of a two-step reaction. The primary substitution reaction was sufficient in 1 min, and the secondary oxidation and substitution reactions were completed within 10 min. As a result, the concentration of 4-chloro-2,6-DMP detected is proportional to the concentration of residual hypochlorous acid/hypochlorite, and the concentration of 4-iodo-2,6-DMP detected is correlated with the concentration of residual chlorine dioxide.
In order to calculate the concentration of two disinfectants, a calibration curve should be prepared using standard materials of the two disinfectants, and the concentration should be obtained from the calibration curve. However, both disinfectants are unstable, so it is not possible to obtain an accurate concentration standard. Therefore, the concentrations of the two disinfectants can be calculated from a calibration curve of two commercially available derivatives.
In the reaction of Scheme I, 1 mole of hypochlorite produces 1 mole of 4-chloro-2,6-DMP. In the reactions of Schemes II and III, 2 moles of chlorine dioxide produce 1 mole of 4-iodo-2,6-DMP. Therefore, the concentrations of the two disinfectants can be calculated from the calibration curve made with the standard materials of 4-chloro-2,6-DMP and 4-iodo-2,6-DMP using the following formula.
Mixed solutions of chlorine dioxide and hypochlorous acid/hypochlorite at concentrations of 1.0, 5.0 and 50 mg/L were reacted according to the experimental procedure, and the stability of the formed 4-chloro-2,6-DMP and 4-iodo-2,6-DMP was tested at room temperature. It was compared with a standard solution stored at 4oC. The derivatives were stable in the chromatography system and at room temperature for about 1 week. After 1 week, the average area of 4-halo-2,6-DMP decreased to more than 15% at room temperature. These derivatives should preferably be quantified within three days after derivatization.
In order to examine the interfering effects of various ions, the method developed was applied to determine 50 mg/L chlorine dioxide and 50 mg/L hypo-chlorite in pure water, which contains 100 mg/L hydrogen peroxide, carbonate, chloride, chlorite, chlorate, sulfate and nitrate ions, respectively. Carbonate, chloride, chlorite, chlorate, sulfate and nitrate did not interfere with the determination of two disinfectants in water. However, hydrogen peroxide reacted somewhat with iodide ions and interfered with the analysis.
HPLC separation of the derivatives was efficient using a non-polar stationary phase. The column was stable over more than one hundred injections without notable change of the separation characteristics. HPLC chromatograms are shown in Fig. 1. Separation of the derivative from the background was very good. There were no extraneous peaks observed in a chromatogram of blank water at the retention times of 5.59, 6.16 and 6.96 min. The column was stable over 100 injections without appreciable change in separation properties. The HPLC chromatograms after derivatization of blank water and spike samples according to the experimental procedure are shown in Fig. 1. Both 4-chloro-2,6-DMP and 4-iodo-2,6-DMP had good peak shapes and no extraneous peaks were observed near their retention times on the chromatogram.
Since this study used two sample pretreatment methods, the validation of target analytes was also presented in two cases, a high concentration range and a low concentration range, as shown in Table 1.
For the high concentration range, the analytes are either measured directly or by diluting the derivatized mixture with acetonitrile to a concentration range of 0.5-100 mg/L. Validation results in the high concentration range are as follows: The line of best fit is y = 7.814x − 0.008 for chlorine dioxide and y = 8.022x− 0.009 for hypochlorous acid/hypochlorite, where x is the analyte concentration (mg/L) and y is the peak area ratio of the analyte to internal standard. The correlation coefficients were demonstrated to be greater than 0.999. The LOD was 0.3 mg/L for chlorine dioxide and 0.2 mg/L for hypochlorous acid/hypochlorite, and the LOQ was 1.0 mg/L for chlorine dioxide and 0.6 mg/L for hypochlorous acid/hypochlorite. The intra-day and inter-day precisions were less than 8% for two analytes and the intra-day and inter-day accuracies were 102 to 106 % for chlorine dioxide, and 102 to 107 % for hypochlorous acid/hypochlorite (Table 1).
On the other hand, in the low concentration range where highly sensitive measurements in μg/L are required, extraction and concentration processes are required. Validation results in the low concentration range are as follows: The line of best fit with a correlation coefficient greater than 0.999 is y = 7.505x − 0.012 for chlo rine dioxide and y = 7.874x − 0.010 for hypochlorous acid/hypochlorite. By the extraction and concentration method, the LOD was 0.004 mg/L for chlorine dioxide and 0.003 mg/L for hypochlorous acid/hypochlorite, and the LOQ was 0.011 mg/L for chlorine dioxide and 0.009 mg for hypochlorous acid/hypochlorite. The intra-day and inter-day precisions were less than 20% for two analytes and the intra-day and inter-day accuracies were 84 to 89% for chlorine dioxide, and 84 to 88% for hypochlorous acid/hypochlorite (Table 1).
Under the names Miracle or Master Mineral Solution or Miracle Mineral Supplement, approximately 25% sodium chlorite liquid was being sold online with a citric acid "activator". Currently, it has been removed from sale, but the manufacturer instructed consumers to drink the sodium chlorite solution after mixing and diluting it with citric acid such as lemon or lime juice to a final concentration of 0.003% (30 mg/L).
The analytical method developed in this study can be applied to determine the chlorine dioxide and hypochlorous acid/hypochlorite produced when sodium chlorite solution is mixed with citric acid, lemon juice, and lime juice. 30 and 100 mg/L sodium chlorite solutions were prepared by dissolving sodium chlorite in 0.1 M citric acid, 0.05 M hydrochloric acid, undiluted lemon juice, and undiluted lime juice. The chlorine dioxide and hypochlorite concentrations of each sodium chlorite solution were measured over time. There was a big difference in the yield of chlorine dioxide and hypochlorous acid/hypochlorite depending on the type of acid and the initial concentration of chlorite. As shown in Table 2, among the four acid activators, 0.1 M citric acid produced the most chlorine dioxide, and lemon juice produced the second most chlorine dioxide. It is well known that citric acid is effective in generating chlorine dioxide from chlorite, and it is believed that chlorine dioxide is produced by the action of citric acid contained in lemon juice. In addition, when prepared with 100 mg/L chlorite, the chlorine dioxide yield was close to 100%, but when prepared with 30 mg/L chlorite, the chlorine dioxide yield was as low as about 35%. As a result, it was observed that the yield decreased as the concentration of chlorite decreased.
The production ratio of chlorine dioxide to hypochlorite was different depending on the type of acid and the initial concentration of chlorite. The ratio of chlorine dioxide to hypochlorite was the highest when 0.1 M citric acid or lemon juice was used, and when 0.1 M hydrochloric acid was used, most hypochlorite was produced. In addition, when prepared with 100 mg/L of chlorite, the ratio of chlorine dioxide to hypochlorite was about 7 times, but when it was 30 mg/L, it was lowered to 2.5 times. The more dilute the concentration, the more hypochlorite is believed to form a favorable chemical equilibrium.
As can be seen from the previous measurements, when chlorite is dissolved in acid, chlorine dioxide and hypochlorous acid are produced. In this case, the ratio of the two oxidizing agents depends on the concentration of chlorite and acid as shown Scheme IV-VI. As a side reaction, the acidic chlorite solution is converted to chlorous acid (HClO2) [22], which is changed to hypochlorous acid (HClO) and chloric acid (HClO3) [23]. Chloric acid is then converted to chlorine dioxide and hypochlorous acid. Combining the three reactions gives the net reaction equation as shown in Scheme VII.
The US Environmental Protection Agency (USEPA) has provided an oral minimum risk level (MRL) of 0.1 mg/kg/day and a reference dose (RfD) of 0.03 mg/kg/day for chlorine dioxide [20,21,24]. Assuming an average human weight of 60 kg and a daily intake of 1.0 L of MMS, the minimum risk level of 6 mg/day and the reference dose of 1.8 mg/day were calculated for chlorine dioxide. If 30 mg/L sodium chlorite is used with 0.1 M citric acid and 1.0 L of the MMS solution is ingested in one day, chlorine dioxide would be ingested at a maximum of 7.8 mg/day, which exceeds both the MRL and RfD. If 100 mg/L sodium chlorite is used with 0.1 M citric acid to prepare an MMS solution, and 1 L of the solution is consumed per day, the chlorine dioxide consumption is 10 times more than the former, exceeding both the MRL and RfD by 10 times and 40 times respectively.
Meanwhile, the US EPA has provided an RfD of 0.1 mg/kg/day for hypochlorite [20,21,24]. Assuming the same method as chlorine dioxide, the reference dose would be 6 mg/day and hypochlorite would be ingested at a maximum of 9.3 mg/day, exceeding the RfD. When 100 mg/L sodium chlorite is used with 0.1 M citric acid to prepare the MMS solution, hypochlorite consumption exceeds the RfD by a factor of 5.5. As the US Food and Drug Administration (FDA) has warned manufacturers of these products, MMS can significantly exceed the MRL and RfD and cause many adverse effects in humans [25,26].
In this study, a method for simultaneously analyzing chlorine dioxide and hypochlorous acid/hypochlorite in water using HPLC-UV was developed. With this method, byproducts were measured by derivatization using different substitution reactivity of hypochlorite and chlorine dioxide with 2,6-DMP. The developed method is sensitive and selective compared to previous chromatographic methods, and is suitable even in the presence of excess ions and in cases where low concentrations of chlorine dioxide and hypochlorite are required, such as in water treatment plants. This method is therefore suitable for the routine analysis of chlorine dioxide and hypochlorite in water or chlorine dioxide products.
Fig. 1.
Chromatogram of the extract of blank (up) and pure water (down) spiked with standard (0.02 mg/L) and internal standard (0.2 mg/L). (A)=4-chloro-2,6-DMP (5.59 min); (B)=4-iodo-2,6-DMP (6.96 min) and (C)=2,4,6-TCP (6.16 min) as internal standard
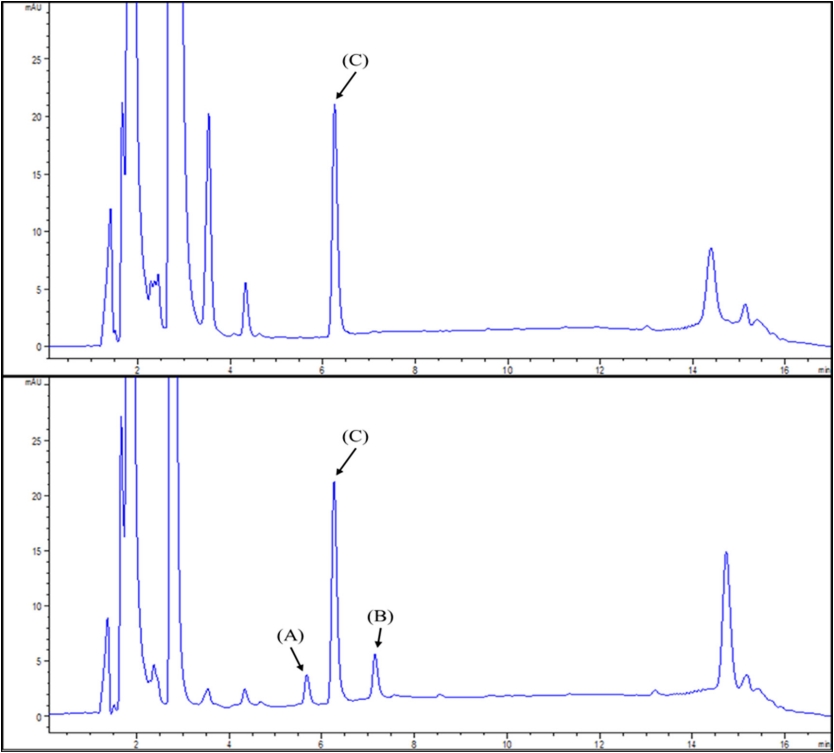
Table 1.
Validation of chlorine dioxide and hypochlorous acid/hypochlorite in water
| Compound* | Conc level |
Detection limits (n=7) |
Calibration curve |
Spiked Conc (mg/L) |
Intra-day measured value (n=5) |
Inter-day measured value (n=3) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD (mg/L) | LOQ (mg/L) | Conc range (mg/L) | Linear equation | Regression coefficient (r2) | Mean±SD (mg/L) | Accuracy (%) | Precision (%) | Mean±SD (mg/L) | Accuracy (%) | Precision (%) | |||
| Chlorine dioxide | **Low conc | 0.004 | 0.011 | 0.005~0.54 | y=7.505x-0.012 | 0.9995 | 0.025 | 0.022±0.004 | 88.0 | 18.2 | 0.021±0.003 | 84.0 | 14.3 |
| 0.100 | 0.089±0.009 | 89.0 | 10.1 | 0.087±0.011 | 87.0 | 12.6 | |||||||
| ***High conc | 0.3 | 1.0 | 0.3-100 | y=7.814x-0.008 | 0.9997 | 5.0 | 5.107±0.211 | 102 | 4.13 | 5.124±0.308 | 103 | 6.01 | |
| 20.0 | 21.077±0.432 | 105 | 2.05 | 21.254±0.516 | 106 | 2.43 | |||||||
| Hypochlorous acid/hypochlorite | **Low conc | 0.003 | 0.009 | 0.004~0.43 | y=7.874x-0.010 | 0.9991 | 0.025 | 0.021±0.004 | 84.0 | 19.1 | 0.022±0.003 | 88.0 | 13.6 |
| 0.100 | 0.088±0.008 | 88.0 | 9.09 | 0.087±0.009 | 87.0 | 10.4 | |||||||
| ***High conc | 0.2 | 0.6 | 0.2-100 | y=8.022x-0.009 | 0.9999 | 5.0 | 5.112±0.234 | 102 | 4.58 | 5.129±0.401 | 103 | 7.82 | |
| 20.0 | 21.077±0.432 | 105 | 2.05 | 21.437±0.604 | 107 | 2.82 | |||||||
Table 2.
The production of chlorine dioxide and hypochlorous acid/hypochlorite over time in mixture solution of sodium chlorite liquid and acids
References
1. E. M. Aieta, and J. D. Berg, “A review of chlorine dioxide in drinking water treatment”, Journal American Water Works Association, 1986, 78 (6), 62-72.


2. Z. Zhang, C. McCann, J. E. Stout, S. Piesczynski, R. Hawks, R. Vidic, and V. L. Yu, “Safety and efficacy of chlorine dioxide for Legionella control in a hospital water system”, Infection Control & Hospital Epidemiology, 2007, 28, 1009-1012.


3. N. Ogata, and T. Shibata, “Protective effect of low-concentration chlorine dioxide gas against influenza A virus infection”, Journal of General Virology, 2008, 89, 60-67.


4. World Health Organization (WHO), "Cleaning and disinfection of environmental surfaces in the context of COVID-19". 2023
5. S. Fukuzaki, “Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes”, Biocontrol science, 2006, 11, 147-157.


6. G. Dev Kumar, A. Mishra, L. Dunn, A. Townsend, I. C. Oguadinma, K. R. Bright, and C. P. Gerba, “Biocides and novel antimicrobial agents for the mitigation of coronaviruses”, Frontiers in microbiology, 2020, 11, 1351.
7. T. Miura, and T. Shibata, “Antiviral Effect of Chlorine Dioxide against Influenza Virus and its Application for Infection Control”, The Open Antimicrobial Agents Journal, 2010, 2, 71-78.
8. N. Hatanaka, B. Xu, M. Yasugi, H. Morino, H. Tagishi, T. Miura, T. Shibata, and S. Yamasaki, “Chlorine dioxide is a more potent antiviral agent against SARS-CoV-2 than sodium hypochlorite”, Journal of Hospital Infection, 2021, 118, 20-26.



9. US Food and drug administration, https://www.fda.gov/consumers/consumer-updates/danger-dont-drink-miracle-mineral-solution-or-similar-products, March 2023
10. US Food and drug administration, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-warns-seller-marketing-dangerous-chlorine-dioxide-products-claim, March 2023
11. X. Kang, and X. Fan, “The use of naphthol green for the determination of chlorine dioxide in water”, Analytical letters, 2003, 36, 1661-1667.

12. X. D. Zhao, and Q. Y. Zeng, “Spectrophotometric determination of chlorine doxide in drinking water by bromocresol purple”, CHINESE JOURNAL OF ANALYTICALCHEMISTRY, 2001, 29, 989-990.
13. R. D. Gauw, G. L. Emmert, B. Bubnis, and G. Gordon, “High resolution spectrophotometry for identification of chlorine dioxide in concentrated chlorine solutions”, Talanta, 1999, 50, 1073-1078.


14. G. Jin, J. Yang, and J. F. Li, “Determination of chlorine dioxide using capillary on-line concentration coupled with flow injection analysis”, Microchimica Acta, 2004, 148, 171-175.


15. D. G. Themelis, and F. S. Kika, “Gas-diffusion flow injection assay for the selective determination of chlorine dioxide based on the fluorescence quenching of chromotropic acid”, Microchemical journal, 2006, 82, 108-112.

16. Z.-L. Jiang, B.-M. Zhang, and A.-H. Liang, “A new sensitive and selective fluorescence method for determination of chlorine dioxide in water using rhodamine S”, Talanta, 2005, 66, 783-788.


17. F. Tian, and J. L. Xie, “Determination of chlorine dioxide, chlorine, chlorite and chlorate in water by ion chromatography”, CHINESE JOURNAL OF ANALYTICAL CHEMISTRY, 2004, 32, 522-524.
18. T. Watanabe, T. Idehara, Y. Yoshimura, and H. Nakazawa, “Simultaneous determination of chlorine dioxide and hypochlorite in water by high-performance liquid chromatography”, Journal of Chromatography A, 1998, 796, 397-400.

19. H.-S. Shin, and D.-G. Jung, “Determination of chlorine dioxide in water by gas chromatography-mass spectrometry”, Journal of Chromatography A, 2006, 1123, 92-97.


20. Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Chlorine Dioxide and Chlorite, 2004.
21. Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Chlorine, 2010.
22. E. Ortenberg, and B. Telsch, “Taste and odour problems in potable water”, Handbook of Water and Wastewater Microbiology, 2003, 777-793.

23. R. C. Ropp, “Group 17 (H, F, Cl, Br, I) alkaline earth compounds”, Encyclopedia of the Alkaline Earth Compounds, 2013, 25-104.

24. US Environmental protection agency, Health Risk Assessment/Characterization of the Drinking Water Disinfection Byproducts Chlorine Dioxide & Chlorite, 1998.
25. The Sydney Morning Herald, https://www.smh.com.au/national/deadly-chemical-being-sold-as-miracle-cure-20100108-lyvl.html, January 2010
26. Ars Technica, https://arstechnica.com/science/2019/08/the-fda-warns-not-to-drink-bleach-in-case-you-needed-that-reminder, August 2019
- TOOLS






