 |
 |
- Search
| J Environ Anal Health Toxicol > Volume 24(3); 2021 > Article |
|
ABSTRACT
Fig. 1.
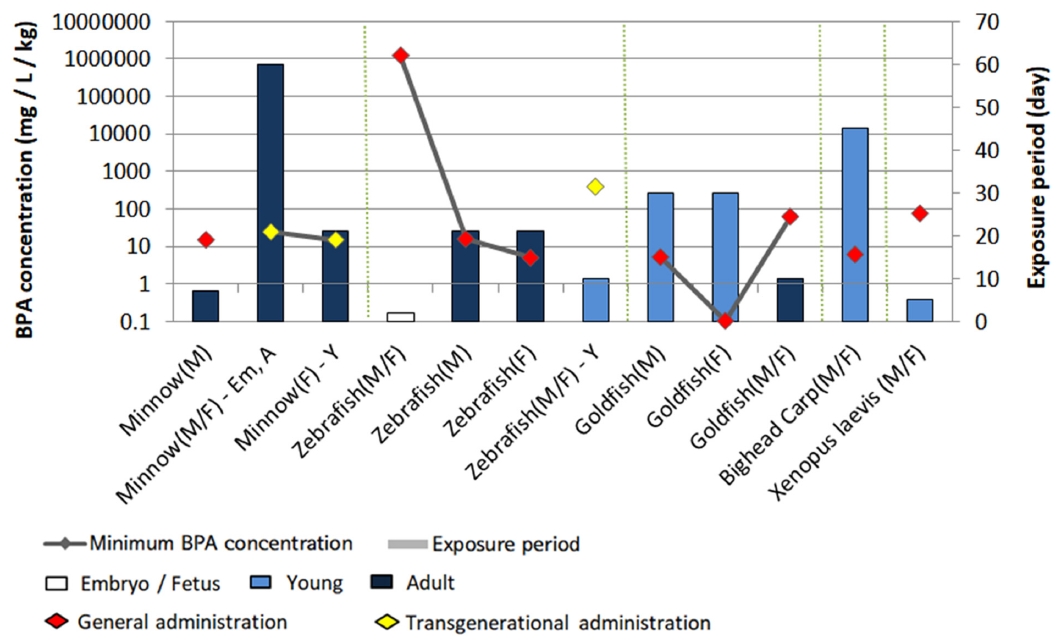
Fig. 2.
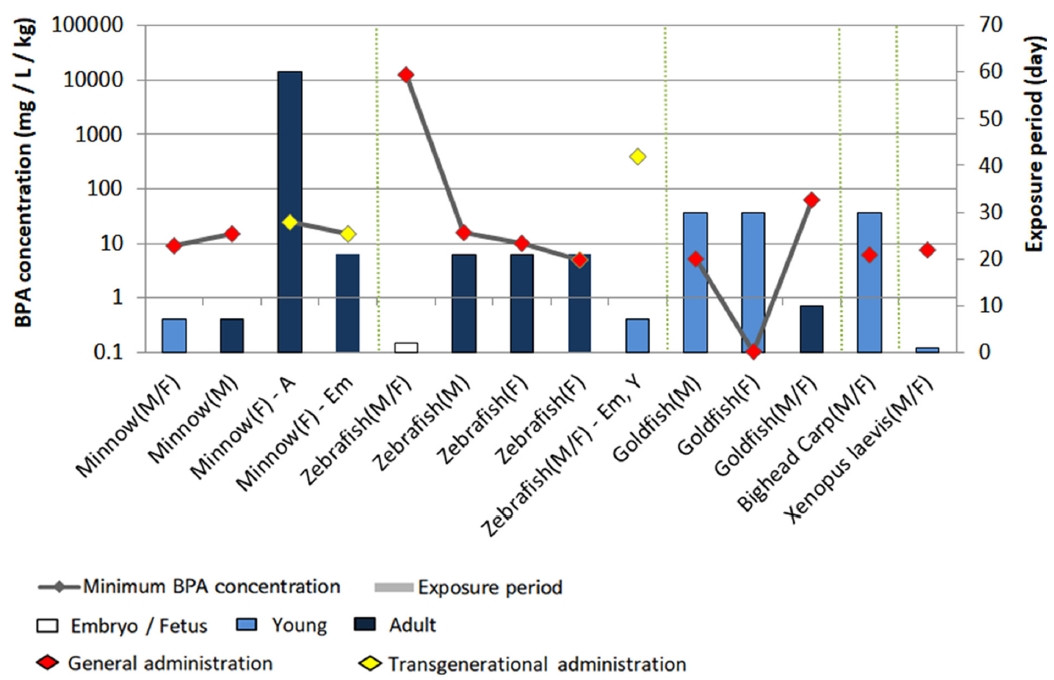
Fig. 3.

Fig. 4.

Table 1.
| Species | Age (treated), Body weight, Sex | Age (observed), Sex | BPA concentration (őľg/L) (duration, method of administration) [minimum toxic concentration/body weight (mg/L/kg)] | (General/Transgenerational) effects |
|---|---|---|---|---|
| Minnow (Gobiocypris rarus) | A (5 months), 1 ¬Ī 0.3 g, M | A (5~6 months), M | 15 (1, 3, 5 weeks, half of the water renewed daily) [15] | (G) Liver became a little whiter and cell arrangement became loose. [34] |
| A (3 months), 0.61 ¬Ī 0.05 g, M/F | Em, M/F | 15 (2 months, half of the water renewed daily, dissolved in DMSO) [24.5902] | (T) Size and number of eggs reduced significantly, and malformation was observed, but eggs from parents with more than 2 months of recovery before mating showed no effect. [33] | |
| A (5 months), F | (T) Vitellogenic oocytes decreased, and cortical alveolus oocytes increased in ovaries. [33] | |||
| A (6 months), 1 g [32], F | Y (48 dpf), M/F | 15, 225 (21 days, half of the water renewed daily) [15] | (T) Egg diameter and bone size decreased. Malformation and developmental defects increased. [31] | |
| Zebrafish (Danio rerio) | Em (2 hpf), 0.08 mg [72], M/F | Y (larvae, 120 hpf), M/F | 100 (118 hours, renewed every 12 hours, dissolved in DMSO) [1250000] | (G) BPA reduced body weight. [29] |
| A (1 year), 0.63 ¬Ī 0.07 g [73], M | A (1 year), M | 10, 20 (3 weeks, dissolved in 100% ethanol) [15.8730] | (G) Gonadosomatic index (GSI = gonad weight/body weight) increased concentration-dependently. The testicular area of spermatogonia was reduced. [27] | |
| A, 1 g ¬Ī 0.16 g [73], F | A, F | 5, 10, 20 (3 weeks, renewed every 4 days) [5] | (G) Hepatosomatic index (HIS = liver weight/body weight) increased but not in higher concentrations. [26] | |
| Y (4 months), 0.58 ¬Ī 0.12 g [73], M/F | Y (larvae, 120 hpf), M/F | 228.29 (7 days, renewed every day, dissolved in DMSO) [393.6034] | (T) Vacuolization occurred in the cytoplasm of hepatic cells. [28] | |
| Goldfish (Carassius auratus) | Y (3 months), 9.6 ¬Ī 0.78 g, M | Y (4 months), M | 50, 500 (30 days) [5.2083] | (G) Testis weight decreased significantly and GSI (gonadosomatic index = 100 √ó gonad weight/body weight) declined. Spermatogonia and spermatocytes disappeared in testes. [36] |
| Y (3 months), 9.6 ¬Ī 0.78 g, F | Y (4 months), F | 1, 50, 500 (30 days) [0.1042] | (G) Cortical alveolar oocytes disappeared, but primary growth oocytes increased. Weights of ovaries decreased significantly. GSI declined but not after 30 days of BPA withdrawal. [36] | |
| A, 36.27 ¬Ī 7.69 g, M/F | A, M/F | 2282.9, 5707.25 (10 days, renewed every day, dissolved in dechlorinated and carbon filtered tap water) [62.9418] | (G) Cardiosomatic index (CSI = 100 √ó heart weight/body weight) increased. [35] | |
| Bighead Carp (Hypophthalmichthys nobilis) | Y, 162.5 ¬Ī 12.5 g, M/F | Y, M/F | 1000, 1500 (45, 60 days, mixed in ethanol) [6.1538] | (G) Significant morphological defects were observed in red blood cells. Broken nuclei were observed. [24] |
| Xenopus laevis | Y (Stage 52 [74]), 0.3 ¬Ī 0.1 g [75], M/F | Y (stage 54 [74]), M/F | 22.829, 228.29 (5 days, renewed 24 hours) [76.0967] | (G) Epithelial folds emerged in intestine. [38] |
1 When the age of exposed parents was not specified in the journal article, we marked it as ‚Äėadult‚Äô in the age column.
2 When the exact age or body weight of the experimental animals were not specified, we inferred it from cited academic articles.
3 BPA concentrations were rounded to four decimal places and numbers of experimental animals’ body weight were rounded to two decimal places.
4 BPA was administered daily except where some other period is stated. When BPA was administered periodically, instead of daily, the duration of administration was recorded.
5 Both the actual BPA concentration used in each experiment and the calculated figures (BPA concentration/average body weight of the experimental animal) were written together in the BPA concentration column.
6 Molar concentration (mol/L) was converted into mass concentration (őľg/L).
7 G: general administration; T: transgenerational administration; Em: embryo; Y: young; A: adult; dpf: days post fertilisation; hpf: hours post fertilisation; DMSO: dimethyl sulfoxide.
Table 2.
| Species | Age (treated), Body weight, Sex | Age (observed), Sex | BPA concentration (őľg/L) (duration, method of administration) [minimum toxic concentration/body weight (mg/L/kg)] | (General/Transgenerational) Effects |
|---|---|---|---|---|
| Minnow (Gobiocypris rarus) | Y (2 weeks), 0.105 g, M/F | Y (3 weeks), M/F | 1, 225, 1000 (7 days, half of the water daily replaced) [9.0909] | (G) Oxidative stress suppressed the immune system of fish larvae. [30] |
| A (5 months), 1 ¬Ī 0.3 g, M | A (5~6 months), M | 15 (1, 3, 5 weeks, half of the water daily replaced) [15] | (G) Upregulated levels of triglyceride induced lipid accumulation in the liver. [34] | |
| A (6 months), 1 g [32], M | A (6~7 months), M | 15 (1, 3, 5weeks, half of the water daily replaced) [15] | (G) Congestion of blood vessels and infiltration of inflammatory cells occurred, but not until after 35 days. The permeability of the Sertoli cell (SC) barrier increased. BPA disturbed the expression of SC junction proteins. [32] | |
| A (3 months), 0.61 ¬Ī 0.05 g, F | A (5 months), F | 15 (2 months, half of the water daily replaced, dissolved in DMSO) [24.5902] | (T) Expression of steroidogenic genes related to ovarian development was disturbed. [33] | |
| A (6 months), 1 g [32], F | Em (48 hpf) | 15, 225 (21 days, half of the water daily replaced) [15] | (T) Ossification was delayed, and embryonic heart rates increased. [31] | |
| Zebrafish (Danio rerio) | Em (2 hpf), 0.08 mg [72], M/F | Y (larvae, 120 hpf), M/F | 1, 100 (118 hours, renewed every 12 hours, dissolved in DMSO) [12500] | (G) BPA affected the reproductive neuroendocrine system by regulating gene expression. [29] |
| A (1 year), 0.63 ¬Ī 0.07 g [73], M | A (1 year), M | 10, 20 (3 weeks, dissolved in 100% ethanol) [15.8730] | (G) Changes in endocannabinoid system (ECS) related genes‚Äô transcript levels were observed. [27] | |
| A (1 year), 1 ¬Ī 0.16 g [73], F | A (1 year), F | 10, 20 (3 weeks, dissolved in 100% ethanol) [10] | (G) Changes in ECS‚Äôs lipid mediators affected the reproductive function of the ovary. [27] | |
| A, 1 ¬Ī 0.16 g [73], F | A, F | 5, 10, 20 (3 weeks, renewed 4 days) [5] | (G) BPA interrupted lipid metabolism which increased the total lipid concentration in the liver. BPA increased the ratio of oocytes in the vitellogenic stage at low concentrations. [26] | |
| Y (4 months), 0.58 ¬Ī 0.12 g [73], M/F | Em (4.5 hpf), M/F | 228.29 (7 days, renewed every day, dissolved in DMSO) [393.6034] | (T) Changes in epigenetic regulation of gene expression affected embryogenesis and early development. [28] | |
| Y (4 months), 0.58 ¬Ī 0.12 g [73], M/F | Y (larvae, 120 hpf), M/F | (T) Changes in epigenetic regulation of gene expression led to oxidative stress, apoptosis, and DNA damage. [28] | ||
| Goldfish (Carassius auratus) | Y (3 months), 9.6 ¬Ī 0.78 g, M | A (4 months), M | 50, 500 (30 days) [5.2083] | (G) Apoptosis of Leydig cells led to a decrease in androgen levels and disruption of testicular spermatogenesis. [36] |
| Y (3 months), 9.6 ¬Ī 0.78 g, F | A (4 months), F | 1, 50, 500 (30 days) [0.1042] | (G) Changes in hypothalamic-pituitary-gonad (HPG) axis-related genes led to immaturity of reproductive organs. [36] | |
| A, 36.27 ¬Ī 7.69 g, M/F | A, M/F | 2282.9, 5707.25 (10 days, renewed every day, dissolved in dechlorinated and carbon filtered tap water) [62.9418] | (G) Oxidative damage increased significantly. [35] | |
| Bighead Carp (Hypophthalmichthys nobilis) | Y, 162.5 ¬Ī 12.5 g, M/F | Y, M/F | 1000, 1500 (30, 45, 60 days, mixed in ethanol) [6.1538] | (G) Severe changes in behavioural signs were observed, and oxidative stress increased. Lower rate of antioxidant enzymes was observed. DNA damage occurred in blood lymphocytes, hepatocytes, brain, gill, and kidney tissues. [24] |
| Xenopus laevis | Y (stage 52 [74]), 0.3 ¬Ī 0.1 g [75], M/F | Y (stage 52 [74]), M/F | 2.2829, 22.829, 228.29 (1 day, dissolved in DMSO) [7.6097] | (G) BPA induced agonistic activity of glucocorticoid signalling by upregulating gene expression. [39] |
| Y (stage 52 [74]), 0.3 ¬Ī 0.1 g [75], M/F | Y (stage 54 [74]), M/F | 2.2829, 22.829, 228.29 (5 days, renewed every day) [7.6097] | (G) BPA disrupted Notch signalling which disturbed the intestinal development of individuals. [38] |
1 When the age of exposed parents was not specified in the journal article, we marked it as ‚Äėadult‚Äô in the age column.
2 When the exact age or body weight of the experimental animals were not specified, we inferred it from the cited academic articles.
3 BPA concentrations were rounded to four decimal places and numbers of experimental animals’ body weight were rounded to two decimal places.
4 BPA was administered daily except where some other period is stated. When BPA was administered periodically with a specific period, instead of daily administration, the duration of administration was recorded.
5 Both the actual BPA concentration used in each experiment and the calculated figures (BPA concentration/average body weight of the experimental animal) were written together in the BPA concentration column.
6 Molar concentration (mol/L) was converted into mass concentration (őľg/L).
7 The 1st young minnow’s body weight is the control’s weight after seven days.
8 G: general administration; T: transgenerational administration; Em: embryo; Y: young; A: adult; hpf: hours post fertilisation; DMSO: dimethyl sulfoxide
Table 3.
| Species | Age (treated), Sex | Age (observed), Sex | BPA dose (mg/kg) (duration, method of administration) | (General/Transgenerational) Effects |
|---|---|---|---|---|
| Japanese quail | Y (3 weeks), M | A (12 weeks), M | 1, 5, 10 (once a week for three weeks, dissolved in corn oil and intraperitoneally injected) | (G) Malformation of testicles was found. BPA interrupted the histological development of the testis. [42] |
| Y (3 weeks), F | A (8, 10, 12, 14 weeks), F | (G) Body weight and weight of reproductive organs increased. [43] | ||
| Em (egg) | (T) Quality (such as shell thickness and shape index) decreased. [43] | |||
| Hyline chicken | Em (ED 10.5), 52.3 g egg weight [76], F | Em (ED 12.5, 15.5, 17.5, 18.5), F | 0.9560, 9.5602 (every other day for ED 10.5~18.5, injected) | (G) The ovarian cortex became thicker, and the size of the germ cell cyst in the ovary increased. [41] |
| Kadaknath chicken | A (25 weeks), M | A (32 weeks), M | 5 (7 weeks, dissolved in DMSO, orally administered) | (G) A significant difference in the total body weight was not observed, but the maximum increase of body weight was higher. [40] |
| CD-1 mice | Y (new-born), F | Y (2 weeks after parturition), F | 0.05, 10 (every 3 days for 60 days, dissolved in 100% ethanol and diluted in corn oil, subcutaneously injected) | (G) Females’ body weight remained high after parturition. [49] |
| Pzh:Sfis mice | Y (4.5 weeks), M | A (8~9 weeks), M | 10, 20 (8 weeks, dissolved in 70% ethyl alcohol, administered in drinking water) | (T) Skeletal anomaly increased dose-dependently. [48] |
| Kunming mice | A (10 weeks), F | Y (PND 21), F | 2.5, 5, 10, 20, 40 (GD 0.5~17.5, dissolved in corn oil and intragastrically administratered) | (T) The ratio of weight of uterus or ovary to body weight increased at low doses. [47] |
| Y (PND 56), F | (T) The ratio of weight of uterus or ovary to body weight increased at specific doses. Ovary became atrophied. [47] | |||
| Wistar rat | Y (PND 25), M | Y (PND 85), M | 0.1 (60 days, gavaged) | (G) More weight gain, darker fur colour, DNA fragmentation in aorta cells, and irregular thoracic aorta formation were observed. [58] |
| A (10 weeks), F | Y (PND 1), M/F | 0.05, 5 (GD 3~18, dissolved in corn oil and orally administered) | (T) Weight of the female individual’s body, liver, heart, and spleen increased. [59] | |
| Y (PND 21), M/F | (T) Brain and liver weight decreased, and body weight increased in both sexes. [59] | |||
| Y (PND 60), M/F | (T) Female individual’s body weight increased, but the weight of the heart and kidney decreased. [59] | |||
| Wistar albino rat | A (3 months), M | A (about 6 months), M | 50, 500, 1000 (90 days, diluted in olive oil and orally administered) | (G) Dose-dependent degeneration in germinal layer of seminiferous tubule was observed. Leydig cells degenerated and intertubular space increased. [60] |
| NCTR CD-SD rat | A (10~14 weeks), F | Y (PND 1), M | 0.0025, 0.025, 0.25, 2.5, 25 (GD 6~21(parturition),77) gavaged) | (T) Body weight increased. Urethra length and urothelium thickness decreased. Angle of colliculus changed, and the shape and size of urethra varied. [56] |
| Sprague Dawley rat | A (4~7 months), F | Fe | 0.5, 5, 50 (GD 1~20, dissolved in corn oil and intraperitoneally injected) | (T) Bone length and ossification area decreased in extremity bones as BPA dose increased. BPA negatively affected bone metabolism and development. [55] |
| Suffolk sheep | A (2~3 years), F | A (2~3 years), F | 0.5 (GD 30~65, dissolved in corn oil and subcutaneously injected) | (G) The number of placentomes in first stage increased and body weights of fetus (embryo) decreased but not in gestation day 90. [70] |
| A (2~3 years), F | A (22 month), F | 0.5 (GD 30~90, dissolved in corn oil and subcutaneously injected) | (T) Lower lung weight was observed. The ratio of kidney to body weight decreased. [68] | |
| Landrace x Yokshire pig | A (pregnant, 211.63¬Ī2.65 kg), F | A, F | 0.5623 [GD 1~115,78) orally administered (average 119 mg per day)] | (G) Placental tissue integrity decreased, and trophoblastic cells of the placenta showed slight cavitation. Placental and litter weights increased. [66] |
| Y (S), M/F | (T) Birth weight increased. [66] | |||
| A (pregnant, 221.14¬Ī2.57 kg), F | Y (S), M/F | 0.5381 [GD 1~115,78) orally administered (average 119 mg per day)] | (T) Dressing percentage and weight of fat around kidneys increased. Colour of muscle changed. [65] |
1 When the age of dosed parents was not specified in the journal article, we marked it as ‚Äėadult‚Äô in the age column. Egg weight of Hyline chicken was inferred from the cited academic article.
2 BPA doses were rounded to four decimal places and numbers of experimental animals’ body weight were rounded to two decimal places.
3 BPA was administered daily except where some other period is stated. When BPA was administered periodically, instead of daily, the duration of the administration was recorded.
4 Both the actual BPA dose used in each experiment and the calculated figures (BPA dose/average body weight of the experimental animal) were written together in the BPA dose column.
5 M: male; F: female; Fe: fetus; Em: embryo; S: before suckling; Y: young; A: adult; G: general effect; T: transgenerational effect; PND: postnatal day; ED: embryonic day; GD: gestation day; DMSO: dimethyl sulfoxide
Table 4.
| Species | Age (treated), Sex | Age (observed), Sex | BPA dose (mg/kg) (duration, method of administration) | (General/Transgenerational) Effects |
|---|---|---|---|---|
| Japanese quail | Y (3 weeks), M | A (12 weeks), M | 1, 5, 10 (once a week for three weeks, dissolved in corn oil and intraperitoneally injected) | (G) Male reproductive capacity was decreased by effects on sperm mobility, foam formation, sperm formation, and the amount of semen. [42] |
| Y (3 weeks), F | A (8, 10, 12, 14 weeks), F | (G) Puberty and the initiation of egg production were delayed. Higher estradiol concentrations were observed. [43] | ||
| Hyliine chicken | Em (ED 10.5, 52.3 g) egg weight [76], F | Em (ED 12.5, 15.5, 17.5, 18.5), F | 0.9560, 9.5602 (every other day for ED 10.5~18.5, injected) | (G) BPA changed the hypomethylation of estrogen receptor ő≤ (ERő≤) signalling pathways, which encouraged meiosis and ovarian germ cell development of oocytes. [41] |
| Kadaknath chicken | A (25 weeks), M | A (32 weeks), M | 1, 5 (7 weeks, dissolved in DMSO and orally administered) | (G) In the low dose group alone BPA affected serum testosterone level and sperm characteristics (semen quantity, sperm concentration) without altering its fertilizing ability. [40] |
| CD-1 mice | Y (new-born), M/F | Y (PND 60, 90), M/F | 0.05, 10 (every 3 days for 60 days, dissolved in 100% ethanol and diluted in corn oil, subcutaneously injected) | (G) Sperm count decreased. The content of estrogen (E2) in plasma increased. In males E2 level increased only in the high dose group. [49] |
| Swiss mice | Y (3 weeks), M/F | Y (PND 60), M/F | 5 (40 days, dissolved in canola oil and intragastrically administratered) | (G) Defects in object recognition memory were observed. Female individuals had defects in spatial memory and male individuals had defects in passive avoidance. Glutamate uptake and levels of N-Methyl-D-aspartate (NMDA) receptor subunits in the cortex and hippocampus decreased, sex-dependently. [50] |
| Kunming mice | Y (4 weeks), M | Y (12 weeks), M | 0.0005, 0.05, 5 (8 weeks, dissolved in tea oil and orally injected) | (G) The rate of DNA-damaged brain cells increased dose- dependently. [46] |
| A (10 weeks), F | A (10~13 weeks), F | 5, 10, 20, 40 (GD 0.5~17.5, dissolved in corn oil and intragastrically administratered) | (G) The abortion rate increased. [47] | |
| A (10 weeks), F | Y (PND 21), F | 2.5, 5, 10, 20, 40 (GD 0.5~17.5, dissolved in corn oil and intragastrically administratered) | (T) BPA altered the expression of apoptosis-related proteins. The plasma and ovarian content of sex hormone and its receptors changed. The number of follicles increased dose-dependently. [47] | |
| Y (PND 56), F | (T) The expression and transcript levels of apoptosis-related proteins changed. The content and transcript levels of sex hormone receptors changed. [47] | |||
| C57BL/6J mice | A (8 weeks), M | A (16~17 weeks), M | 0.04, 0.4 (60 days, dissolved in corn oil and orally administered) | (G) Motor skills, coordination and balance declined. Myelin and axonal degeneration occurred in the oligodendrocyte. [52] |
| Pzh:Sfis mice | Y (4.5 weeks), M | A (8~9 weeks), M | 5, 10, 20 (8 weeks, dissolved in 70% ethyl alcohol, administered in drinking water) | (T) Sperm mortality decreased and average male to female ratio changed. [48] |
| OF-1 mice | A (pregnant), F | Y (PND 30), M | 0.01, 0.1 (GD 9~16, dissolved in corn oil and subcutaneously injected) | (T) BPA changed pancreatic function related to all-trans-retinoic acid (ATRA) metabolism. Also, BPA altered the progress of glucose metabolism. [51] |
| Wistar rat | Y (PND 25), M | A (PND 85), M | 0.1 (60 days, gavaged) | (G) Experimental animals became more aggressive, irritated, and showed piloerection. More reactive oxygen species (ROS) induced higher blood pressure and lower vascular reactivity. Reactivity towards acetylcholine declined. [58] |
| A (7 weeks), M | A (11~12 weeks), M | 0.5, 5, 50 (30 days, dissolved in ethyl alcohol and diluted in sesame oil, orally administered) | (G) Levels or folding of protein and phosphoprotein, related to hepatotoxicity, fatty liver, and carcinoma, changed in low dose. BPA induced liver’s oxidative stress. [57] | |
| A (10 weeks), F | Y (PND 1), M/F | 0.05, 5 (GD 3~18, dissolved in corn oil and orally administered) | (T) Transcript factors and hepatic proteome changed. BPA inhibited male’s androgen receptor (AR). Transcriptome pathway was enriched and hepatic transcriptome changed. [59] | |
| Y (PND 21), M/F | (T) Masculinization of hepatic transcriptome was observed. Hepatic proteome changed and the transcriptome pathway was enriched. [59] | |||
| Y (PND 60), M/F | (T) Plasma cholesterol levels changed. [59] | |||
| Albino rat | A (8 weeks), M | A (14 weeks), M | 50 (3 days a week for 42 days, dissolved in corn oil and orally gavaged) | (G) Decreases in acetylcholine esterase (AChE) activity and cognitive performance ability were observed. Hippocampus showed oxidative stress and neuronal apoptosis. [61] |
| NCTR-SD rat | A (pregnant), F | Y (PND1), M/F | 0.0025, 0.025, 0.25, 2.5, 25 (GD 6~21(parturition), [77] orally administered) | (T) Sex-specific estrogen receptor expression, transcription, and oxytocin expression were observed at the hypothalamus and hippocampus. [53] |
| (T) BPA altered the transcriptome of neonate amygdala; more so in female individuals. Also, BPA interfered with the signalling pathways of vasopressin, oxytocin, synaptic organization, and transmission in the developing brain. [54] | ||||
| Suffolk sheep | A (2~3 years), F | A (2~3 years GD 65), F | 0.5 (GD 30~65, dissolved in corn oil and subcutaneously injected) | (G) Placental efficiency was reduced but not in gestation day 90. Increased inflammation, oxidative stress and decreased insulin-like growth factor (IGF) bioavailability were observed. [70] |
| A (2~3 years GD 90), F | 0.5 (GD 30~90, dissolved in corn oil and subcutaneously injected) | (G) BPA delayed the response to antioxidants which negatively affected placental function. Triglyceride and collagen accumulation occurred at the placenta but not in gestation day 65. [70] | ||
| A (22 months), F | (T) Atrial natriuretic peptide (ANP) increased in the left and right ventricles. [68] | |||
| A (2~5 years), F | A (21 months), F | 0.05, 0.5, 5 (GD 30~90, dissolved in corn oil and subcutaneously injected) | (T) Increased oxidative stress was observed. The expression of estrogen receptors increased in visceral adipose tissues. [67] | |
| Piétrain x Duroc pig | Y (8 weeks), F | Y (12weeks), F | 0.05, 0.5 (28 days, orally administered in capsule form) | (G) The number of neurons, and immunoreaction changed in the duodenum.62) The neurochemical characteristics of uterine sympathetic nerves changed. [63] |
| Landrace x Yorkshire pig | A (pregnant, 211.63¬Ī2.65 kg), F | A, F | 0.5387 [GD 1~35, orally at diet (average 114 mg BPA every day)] | (G) Catalase activity declined which increased oxidative stress in sows. [66] |
| Y (S), M/F | 0.5623 [GD 1~115, [78] orally administered (average 119 mg per day)] | (T) Increased oxidative stress was observed. [66] | ||
| A (pregnant, 221.14¬Ī2.57 kg), F | Y (S), M/F | 0.5381 [GD 1~115, [78] orally administered (average 119 mg per day)] | (T) The muscle content of glycogen and lactate increased, as did lactate dehydrogenase (LDH) activity. [65] |
1 When the age of exposed parents was not specified in the journal article, we marked it as ‚Äėadult‚Äô in the age column. Egg weight of Hyline chicken was inferred from the cited academic article.
2 BPA doses were rounded to four decimal places and numbers of experimental animals’ body weight were rounded to two decimal places.
3 BPA was administered daily except where some other period is stated. When BPA was administered periodically with a specific period, instead of daily administration, the duration of administration was recorded.
4 Both the actual BPA dose used in each experiment and the calculated figures (BPA dose/average body weight of the experimental animal) were written together in the BPA dose column.
5 M: male; F: female; Fe: fetus; ED: embryonic day; Em: embryo; S: before suckling; Y: young; A: adult; G: general effect; T: transgenerational effect; PND: postnatal day; GD: gestation day; DMSO: dimethyl sulfoxide
žįłÍ≥†Ž¨łŪóĆ
- TOOLS






